
Report by Paula Antolini, May 26, 2021 1:26PM EDT
It is very important to stay informed about the ever changing information regarding COVID-19 vaccines presently. Did you know there is online data readily available to the public that has detailed information about adverse reactions from vaccines?
buy Pepcid online https://rxbuyonlinewithoutprescriptionrx.net no prescription
If you are searching for information about symptoms from vaccines, the Vaccine Adverse Event Reporting System (VAERS), offers extensive data you can view and download, that shows vaccine name, manufacturer, and patient’s state, date, age, gender, symptoms, and other information.
buy sertraline online https://rxbuyonlinewithoutprescriptionrx.net/sertraline.html no prescription
— 1,034,476 REPORTS OF VACCINE ADVERSE EVENTS IN VAERS
— 12,993 DEATHS
— 89,848 Hospitalizations
— 227,805 COVID Vaccine Adverse Event Reports*
— 31 YEARS AS OF JAN. 11, 2021
(COVID Data (2020-2021) is updated weekly. The entire dataset from VAERS (1990-2019)is updated yearly.)
*****
What is VAERS?
“Established in 1990, the Vaccine Adverse Event Reporting System (VAERS) is a national early warning system to detect possible safety problems in U.S.-licensed vaccines. VAERS is co-managed by the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA). VAERS accepts and analyzes reports of adverse events (possible side effects) after a person has received a vaccination. Anyone can report an adverse event to VAERS. Healthcare professionals are required to report certain adverse events and vaccine manufacturers are required to report all adverse events that come to their attention,” according to VAERS.
buy strattera online https://rxbuyonlinewithoutprescriptionrx.net/strattera.html no prescription
“The primary objectives of VAERS are to:
- Detect new, unusual, or rare vaccine adverse events;
- Monitor increases in known adverse events;
- Identify potential patient risk factors for particular types of adverse events;
- Assess the safety of newly licensed vaccines;
- Determine and address possible reporting clusters (e.g., suspected localized [temporally or geographically] or product-/batch-/lot-specific adverse event reporting);
- Recognize persistent safe-use problems and administration errors;
- Provide a national safety monitoring system that extends to the entire general population for response to public health emergencies, such as a large-scale pandemic influenza vaccination program.
*****
Here is a section of what one of the numerous data charts looks like, view link below to view all charts on full.
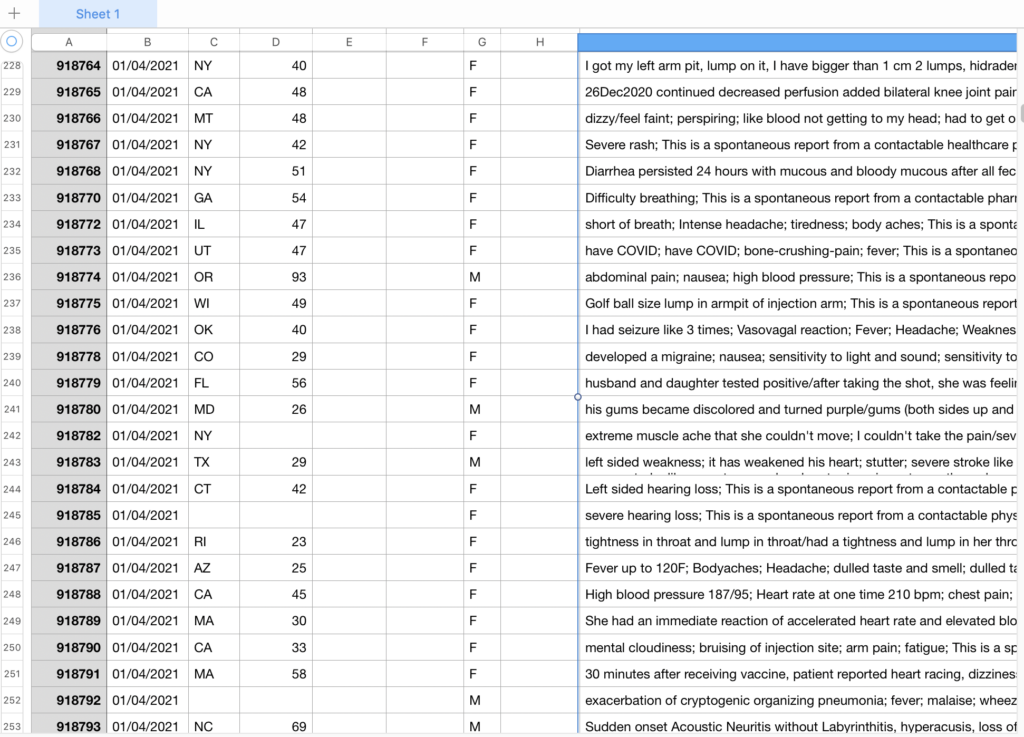
The data on the chart above shows symptoms such as the following (random sample):
On 1-1-21 a 29 year old female from MN, #916742, experienced the following:
“Within 15 minutes of receiving the vaccine I began to get very itchy and blotchy with a hoarse voice. The paramedic downstairs walked me up to the emergency room. I was treated with medications to help calm the itching and burning feeling. By 940 I went anaphylactic and had several doses of epinephrine to help calm this. I continued to have rashes and the feeling of my throat closing. I was transferred by ambulance to medical center in the ICU. I am still here and have had two toner anaphylactic episodes since. I have been on a epi drip, steroids, famotidine, Ativan and Benadryl. I also had a picc like placed.”
On 1-1-21 a 82 year old male from LA, #917026, experienced the following:
“12/28/2020, Pharmacy staff administered Moderna COVID Vaccine. 12/29/2020, he had not eaten breakfast or lunch but did consume fluids and take his medications. BP =150/70, Temp. = 101.6, Pulse= 102, Respirations= 18 and Oxygen saturation= 97%. Tylenol 650 mg given. It was difficult for him to swallow. Also had no use of right upper extremity and unable to move lower extremity, mouth was drooping and was drooling. Physician in attendance and ordered to send to ER. 1/1/2021, received information from nurse at hospital that patient received a Peg Tube this afternoon and Clinical indication of a stroke.”
On 1-1-21 a 26 year old female from CT, #918197, experienced the following:
“Arm soreness at injection site, worse than a flu shot and starting an hour after the shot and lasting approx 2 days. Increased phelgm production for approx 24 hours following injection.”
On 1-4-21 a 42 year old female from CT, #918784, experienced the following:
“Left sided hearing loss; This is a spontaneous report from a contactable physician who was also the patient. A 42-year-old non-pregnant female patient received bnt162b2, lot number and expiration date were unknown, via an unspecified route of administration on 16Dec2020 , 8:30 at a single dose for covid-19 immunization. The patient’s medical history included antiphospholipid antibody from an unknown date and unknown if ongoing. The patient’s concomitant medications were not reported. On 22Dec2020, the patient experienced left sided hearing loss. It was unknown if patient received treatment for the event. The outcome of the event was not recovered. The patient was not diagnosed with COVID prior to vaccination and has not been tested for COVID-19 since vaccination. Patient has no allergies. Patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine. Information on the lot/batch number has been requested.; Sender’s Comments: As an individual case report there is not enough evidence to establish a causal relationship with the suspect vaccine. Currently there is no clear biological plausibility between the vaccine use and the even onset. More information such as complete medical history and concomitant medications are needed for fully medical assessment. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.”
*****
You can also view a video about VAERS below. They mention that the vaccines are AUTHORIZED for use, which means authorized ONLY in an emergency situation, but they fail to mention all vaccines are not APPROVED by the FDA, via proper long-term testing, etc. They weighed “risk” against not having any other methods to stop COVID, and decided to move forward with unapproved vaccines. YOU are therefore left to decide what risk YOU are willing to take. The VAERS website will help you to decide on your choice, after you view symptoms people have experienced
.
*****
Presently there are problems with mandatory vaccine mandates in businesses and schools, including colleges. Some states have legislation to protect citizens’ right, others do not, and individuals are finding themselves in the position of deciding between their jobs or their health, or a student’s education or their health. Many people are fighting back and/or have gone into litigation, or joined groups of like-minded citizens, to fight for their right to choose regarding their health or the health of their loved ones. Read our article here.
There is also the issue of the CDC changing the manner in which they record COVI-19 information, regarding thresholds. Read our article here.
*****
We found that the VAERS system has its drawbacks regarding post-marketing surveillance and other issues. Citizens appear to not be getting the full picture of the vaccine symptoms, and only see the tip of the iceberg with the VAERS system, or they are not being informed about the aftermath of what happens to patients that are affected negatively by the vaccines.
“Robert F. Kennedy, Jr., chairman and chief legal counsel for Children’s Health Defense, is asking Dr. David Kessler, one of the newly named co-chairs of President-Elect Biden’s Transition COVID-19 Advisory Board, to consider the long-overdue need for a comprehensive, high-integrity system to monitor adverse outcomes following vaccination,” according to an article in The Defender, a children’s health and defense publication.
Kennedy said, “Regulatory officials can’t count on post-marketing surveillance to reveal COVID-19 vaccine injuries because the Vaccine Adverse Event Reporting System (VAERS) is broken.”
In a letter written by Robert F. Kennedy, Jr. to David Kessler, MD, JD, on December 18, 2020, regarding “Post-Marketing Surveillance of COVID-19 vaccines” Kennedy said, “COVID-19 vaccines are intended for more Americans than any prior vaccine. As a result, shortcomings in the existing vaccine injury surveillance system will be even more obvious.”
Kennedy said:
1. Regulatory officials cannot count on post-marketing surveillance to reveal COVID-19 vaccine injuries because VAERS is broken.
2. The Department of Health and Human Services has ignored numerous opportunities to strengthen VAERS.
3. Although new post-licensure surveillance efforts have emerged over the past decade, they, too, have extensive limitations.
4. Because COVID-19 vaccines are intended for more Americans than any prior vaccine, COVID-19 vaccination promises to bring existing surveillance systems’ shortcomings into even sharper relief.
Kennedy summarizes:
“As you aptly noted almost three decades ago, lack of reporting delays problem detection. CDC researchers agree that surveillance systems that “detect adverse events as soon as possible after the introduction of new vaccines” are essential to “speed the recognition of increased rates of adverse events” and “inform public policy.” VAERS’s 30-year-old deficits and the non-transparency of systems like the Vaccine Safety Datalink and PRISM/Sentinel are likely to hinder HHS’s [The U.S. Department of Health and Human Services] ability to rapidly identify emerging safety issues with COVID-19 vaccines. These lacunae represent, therefore, a direct threat to patient life and public health. Your committee should not allow further rollout of COVID-19 vaccines until FDA’s capacities for monitoring long-term vaccine safety are significantly improved.”
*****
Pro-vaccine groups
Pro-vaccine groups such as “Voices for Vaccine” claim that, “The use of VAERS reports is an anti-vaccine strategy to spread misinformation.” They recently said, “First of all, let’s emphasize that VAERS is a useful tool, but not for folks looking to confirm their worst fears about vaccines. It’s mostly for the nerds in public health to detect super, super, super rare side effects as early as possible.”
Voices for Vaccines also dismisses a Harvard study, and said, “…the Harvard study says only 1% of vaccine side effects get reported! If you like to nerd out about EMR use from 2007-2010, this study (the source of this misinfo) is great. But it doesn’t say what antivaxxers think it says.”
Their mission: “Voices for Vaccines is a parent-led organization that supports and advocates for on-time vaccination and the reduction of vaccine-preventable disease. Although the majority of parents choose to immunize their children against disease, most of us do not speak out about our decisions because it seems to us common sense. Unfortunately, increasing numbers of parents are choosing not to immunize or to under-immunize their children, which has led to increasing rates of vaccine-preventable diseases and needless suffering.
This method of presenting their viewpoint is possibly misinformation itself, such as stating “vaccine preventable disease” and describing getting a vaccine (including the COVID-19 vaccines) as “common sense” or saying parents who choose to NOT get the vaccine for their children as, “under-immunizing their children.” None of that has been proven.
Also, the COVID-19 vaccine companies all claim the vaccine does not prevent or cure COVID-19, read their fine print.
The FDA has not approved any vaccine, they have just authorized the vaccines for emergency use based on risk. So, to make this into an issue to get vaccinated or not, is NOT the issue. We are not talking about APPROVED COVID-19 vaccines that were well tested.
The issue is whether or not an individual chooses to receive a vaccine or not, based on evaluating vaccine effects that have occurred (because years of testing have not been done) or deciding what they want to put into theor bodies, or that they want to take that risk for themselves or their loved ones at all. They should not be forced into it or be made to feel guilty of they choose not to receive a vaccine.
Also, after reading about devastating affects as in the VAERS reports, or even in local recent news stories of negative vaccine effects, individuals should be able to freely decide on getting a vaccine or not.
These recent stories include incidents in Connecticut where “at least 18 teens and young adults in Connecticut have shown symptoms of heart problems after receiving the COVID-19 vaccine, acting health commissioner Dr. Deirdre Gifford said Monday.” as reported by NBC News.” This report included a follow up story from a 16-year-old high school student from Lyme, CT, Luke Celic, who had heart problems after a 2nd Dose of the Covid-19 vaccine. NBC CT reported, “Celic received his second dose of the Pfizer vaccine on May 10. Three days later, he experienced a very scary occurrence. “My heart started beating really hard. My chest, the center of my chest, just started pounding with sharp pain,” he said. Luke’s mom, a registered nurse, said he came to her in the middle of the night with crushing chest pains and palpitations. “It was difficult for him to catch his breath. He was like panicking. We both didn’t know what was happening,” said Sarah Foley.” … “Foley took her son to an urgent care center, where she said they weren’t sure what was happening.” … “Luke was then airlifted to Yale New Haven Health. He spent three days there in the pediatric ICU and was diagnosed with acute Pericarditis and Myocarditis.” Read more here.
NBC CT reports, “The connection between this and the Covid-19 vaccine, however, has yet to be definitely determined and is currently being evaluated by the CDC. They have not decided or announced that they think there is a relationship but they’re monitoring to see whether this is related to the vaccine,” Deidre Gifford, the acting commissioner of the state Department of Public Health, said.”
There are also individual rights guaranteed by laws and the Constitution.
So for groups such as Voices for Vaccines, and others, to shame people into taking the vaccine because it is “common sense” for instance, is irresponsible and misleading.
*****
In the end it is up to you to do your research and choose what is right for you.
###
