
Report by Paula Antolini, March 24, 2021, 10:51AM EDT
“Our founding fathers fought against tyranny to realize the dream of a great and free nation. It is time for the free people of the greatest nation on earth to stand up for our freedom to choose what’s healthy for our own bodies, before the industrial agriculture and pharmaceutical industries pass laws that force those decisions upon us.
~ Del Bigtree (Founder of ICAN / Informed Consent Action Network)
The Informed Consent Action Network (ICAN) Replies to the FDA’s Deficient Responses to Its COVID-19 Vaccine Citizen Petitions Calling for More Stringent Safety and Efficacy Requirements
ICAN filed a number of Petitions with the FDA demanding that it require certain standards and valid endpoints for determining safety and efficacy in the COVID-19 vaccine trials currently being run by Pfizer, Moderna, Johnson & Johnson, and AstraZeneca. A number of ICAN’s demands, including that the trials be conducted using a placebo control group, came to pass. However, despite the importance of some of ICAN’s other demands, the FDA has now formally denied them and ICAN is pushing back. buy asacol online https://buynoprescriptiononlinerxx.net/asacol.html no prescription
ICAN’s legal team has been working steadfastly on the safety and efficacy requirements for the clinical trials and post-marketing surveillance of COVID-19 vaccines, including demanding that the trials be placebo-controlled, use reliable endpoints and testing, and have other safeguards. Many of those demands were subsequently met but others have now been formally denied.
ICAN’s legal team, led by Aaron Siri, has now pushed back on the FDA’s inadequate and unacceptable responses to the safety and efficacy requirements demanded by ICAN.
Thirteen days after ICAN’s initial petition was filed demanding placebo-controlled trials, the FDA released a guidance document providing that the trials should include a placebo-controlled group. ICAN has now requested that the FDA confirm that the placebo arm will be maintained through the conclusion of the trial in order to maintain the integrity of the trial and the validity of the safety data produced by the trial. ICAN has also requested more comprehensive tracking of adverse events, larger sample sizes within the trials (especially for the pediatric population), and testing of T-cells pre- and post-vaccination. The FDA denied these requests and ICAN has followed-up to let the agency know this is unacceptable given the novel nature of these mRNA vaccines and the current unknowns related to safety. buy atarax online https://buynoprescriptiononlinerxx.net/atarax.html no prescription
In a separate petition, ICAN demanded that the FDA also require that reduction of serious cases of COVID-19 and the prevention of infection and transmission be primary endpoints in the COVID-19 vaccine trials. Many Americans have been led to believe that the vaccines now in use are the answer to all pandemic-related problems. Many believe this is because a vaccine will prevent individuals from becoming infected with SARS-CoV-2 or having a serious case of COVID-19 and will stop people from spreading the virus to others. However, the clinical trials for Pfizer, Moderna, AstraZeneca, and Johnson & Johnson’s products are not designed to determine these things!
This is why ICAN does not accept of the FDA’s refusal to require the vaccine manufacturers to determine whether the vaccine prevents infection and transmission as a primary endpoint. If the FDA does not heed ICAN’s warnings and suggestions, this means that, under the current rules, a COVID-19 vaccine can be fully licensed without demonstrating that it can prevent severe COVID-19, hospitalization, or deaths, nor stop the spread of COVID-19. buy avapro online https://buynoprescriptiononlinerxx.net/avapro.html no prescription
Understanding the unreliability of PCR tests, we further demanded that only positive PCR results meeting certain criteria be relied upon in the clinical trials to determine efficacy. The FDA has refused to change its current stance which is to allow the “sponsor” (the manufacturer) itself to “ensure” that the PCR tests used within the trial are reliable. This is, in effect, the FDA allowing the fox to guard the henhouse.
These alarming deficiencies that still exist in the trials are what led ICAN to direct its attorneys to submit replies to the FDA’s responses and to continue to follow through with demands that will benefit the public. Below is a listing of the Citizen Petitions, FDA responses, and ICAN replies (VIEW LINKS UNDER CHART):
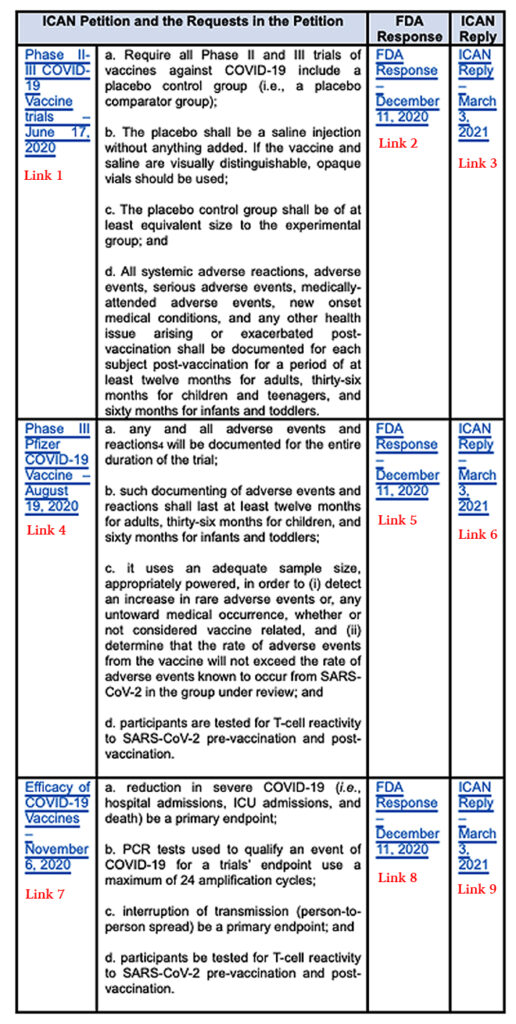
VIEW LINKS CORRESPONDING TO CHART, BELOW:
LINK 1: Phase II-III COVID-19 Vaccine trials – June 17, 2020
LINK 2: FDA Response – December 11, 2020
LINK 3: ICAN Reply – March 3, 2021
LINK 4: Phase III Pfizer COVID-19 Vaccine – August 19, 2020
LINK 5: FDA Response – December 11, 2020
LINK 6: ICAN Reply – March 3, 2021
LINK 7: Efficacy of COVID-19 Vaccines – November 6, 2020
LINK 8: FDA Response – December 11, 2020
LINK 9: ICAN Reply – March 3, 2021
There continues to be numerous other legal and non-legal efforts ICAN is engaged in with regard to COVID-19 vaccines that will be shared in future updates.
*****
ABOUT ICAN
Our Mission:
At the Informed Consent Action Network, you are the authority over your health choices and those of your children. In a medical world manipulated by advertising and financial interests, true information is hard to find and often harder to understand. Our goal is to put the power of scientifically researched health information in your hands and to be bold and transparent in doing so, thereby enabling your medical decisions to come from tangible understanding, not medical coercion.
Who We Are:
Investigating the safety of medical procedures, pharmaceutical drugs, and vaccines while educating the public of their right to “informed consent.”
- FIGHTING FOR PARENTS’ RIGHTS
- PROTECTING CHILDREN
- SCIENCE-BASED INQUIRY
- REASONABLE REQUESTS
- CHILDREN FIRST
- DOCTOR-FRIENDLY
- The Informed Consent Action Network, EIN: 81-4540235, is Texas nonprofit with IRS Section 501(c) (3) taxexempt status.
- Informed Consent Action Network, 2025 Guadalupe Street, Suite 260 Austin, Texas 78705
###
